Product Center
TEBAOLUO
Organic Wastewater Treatment Solution Customization Expert
Organic Wastewater Treatment Solution Customization Expert

Introduction:
There are two methods for crystallization of high brine: one is thermal crystallization, which is suitable for substances whose temperature has little effect on solubility. The “sunshine dry salt” in coastal areas is the method used. The other one is cooling the hot saturated solution method. This method is applicable to substances with increased temperature and increased solubility.There are two methods for crystallization of high brine: one is thermal crystallization, which is suitable for substances whose temperature has little effect on solubility. The “sunshine dry salt” in coastal areas is the method used. The other one is cooling the hot saturated solution method. This method is applicable to substances with increased temperature and increased solubility.For example, in the salt lakes of the northern region, there is no crystal on the lake surface when the temperature is high in summer.When the temperature is lowered in winter, the mirabilite and other substances are separated from the salt lake.
Evaporation Thermal Crystallization
Theory
Evaporating the solvent will cause the solution to change from unsaturated to saturated.If evaporation continues,the excess solute will crystallize out.This is called evaporative crystallization. For example, when there is more NaCl than KNO3 in the mixture of NaCl and KNO3, this method can be adopted. NaCl will be separated first, and KNO3 later.
When the solubility increases with the increase of temperature by observing the solubility curve,, the solute is steep rise type.On the contrary it is slow rise type.
When a steep rise type is mixed with a slow rise type, cooling crystallization can be adopted to separate the steep rise substance.However, evaporation crystallization method separate the slow rise substance.Evaporation crystallization is suitable for substances whose solubility does not change much with temperature, such as sodium chloride.
Potassium nitrate is a steep type, and sodium chloride is a slow rise type. Therefore, it is possible to separate sodium chloride by evaporation crystallization, or to separate potassium nitrate by cooling crystallization.

Roots blower is a positive displacement blower. A certain volume of gas is sucked into the cylinder first. Then its volume is forced to shrink in the cylinder. The gas molecules are close to each other. The density of the gas in the unit volume increases, and the pressure rises. When a certain pressure is reached, the gas is forced out of the cylinder.

As a new technology for the treatment of refractory pollutants, advanced oxidation technology has the characteristics of producing a large amount of very active hydroxyl radicals (·OH), strong oxidizing ability, cleaned degradation products, easy control of reaction process and flexible processing. At present, the advanced oxidation treatment methods commonly used are Fenton and Fenton-like oxidation and ozone oxidation.

The process of removing or reducing calcium or magnesium salts in the raw water is called hard water softening. Softening methods mainly include chemical softening method and ion exchange method.

There are several treatment methods for high-concentration fluorine-containing wastewater at home and abroad, and there are two common methods:adsorption and precipitation. The precipitation method is mainly applied to the treatment of industrial fluorine-containing wastewater, and the adsorption method is mainly to treat drinking water. There are also methods such as freezing, ion exchange, ultrafiltration, fluorine removal, electrocoagulation, electrodialysis, and reverse osmosis.

Air floatation is an alternative method of sedimentation. It forms highly dispersed microbubbles to adhere the solid or liquid particles of hydrophobic groups in wastewater, then forming a water-gas-particle three-phase mixture. In the system, after the particles adhere to the bubbles, a floc having an apparent density smaller than that of water is formed to float to the surface of the water, and the scum layer is scraped off, thereby realizing a process of solid-liquid or liquid-liquid separation.

The flocculation and sedimentation treatment uses a flocculant to cause a process of coagulation and sedimentation of suspended particles in water.

Zero discharge of wastewater means that no waste liquid is discharged from the factory after wastewater treatment process.the pollutants in the salt containing wastewater will be separated and treated and the effluent will be completely reused.The salt and pollutants in the wastewater will be sent to the waste disposal plant in solid form or taken as useful chemical raw materials after being concentrated and crystallized.

The reverse osmosis process is the reverse process of the infiltration.It use the pressure of semi-permeable membrane to separate.The solvent can pass through the semipermeable membrane but the solute can not.

Mine water refers to the external drainage of the mining process. During the mining process, a series of physical, chemical and biochemical reactions occur between the groundwater and the ore layer and the rock formation. The toxic substances in the ore layer and rock formation enter the mine water. If directly discharged without treatment,the mine water will pollute the water body and destroy the ecological environment.In this case,it is necessary to treat the mine water; Affected by the environment, climate or region, mine waters are various in different mine, and even different areas of one mine.Choosing the mine water treatment method according to local conditions can improve the utilization rate and treatment effect of mine water.

Reclaimed water means that the industrial wastewater is treated by high technology to remove various impurities, toxic and harmful substances and some heavy metal ions from polluted water bodies, then the water will be disinfected and sterilized. The water body will be colorless, odorless and clear and transparent. The water will meet or is better than the national standards for miscellaneous water (or related regulations) .This method is widely used in enterprise production.

Electrodialysis is to use selective permeability of the ion exchange membrane to separate the electrolyte from the solution driven by the potential difference under the action of DC electric field, thereby achieving the purpose of desalination, concentration, purification of the solution. The core component of electrodialysis is the membrane stack. The membrane stack is mainly composed of electrodes and ion exchange membranes. The adjacent ion exchange membranes are separated by partitions.

MVR is the abbreviation of mechanical vapor recompression. It uses the secondary steam generated by the evaporation system and upgrade the low-grade steam to a high-grade steam through the mechanical work of the compressor.The high-grade steam will become heat source for evaporation. It supply heat to the evaporation system circularly, thereby reducing the need for external energy.

In the evaporation production, there is a large output of secondary steam that contains huge amount of latent heat.These steam should be recycled for use. The secondary steam can still heat the material when it is passed into another evaporator as long as the operation pressure and boiling point of the latter evaporator is lower than that of the former evaporator.This operation mode is multi-effect evaporator.

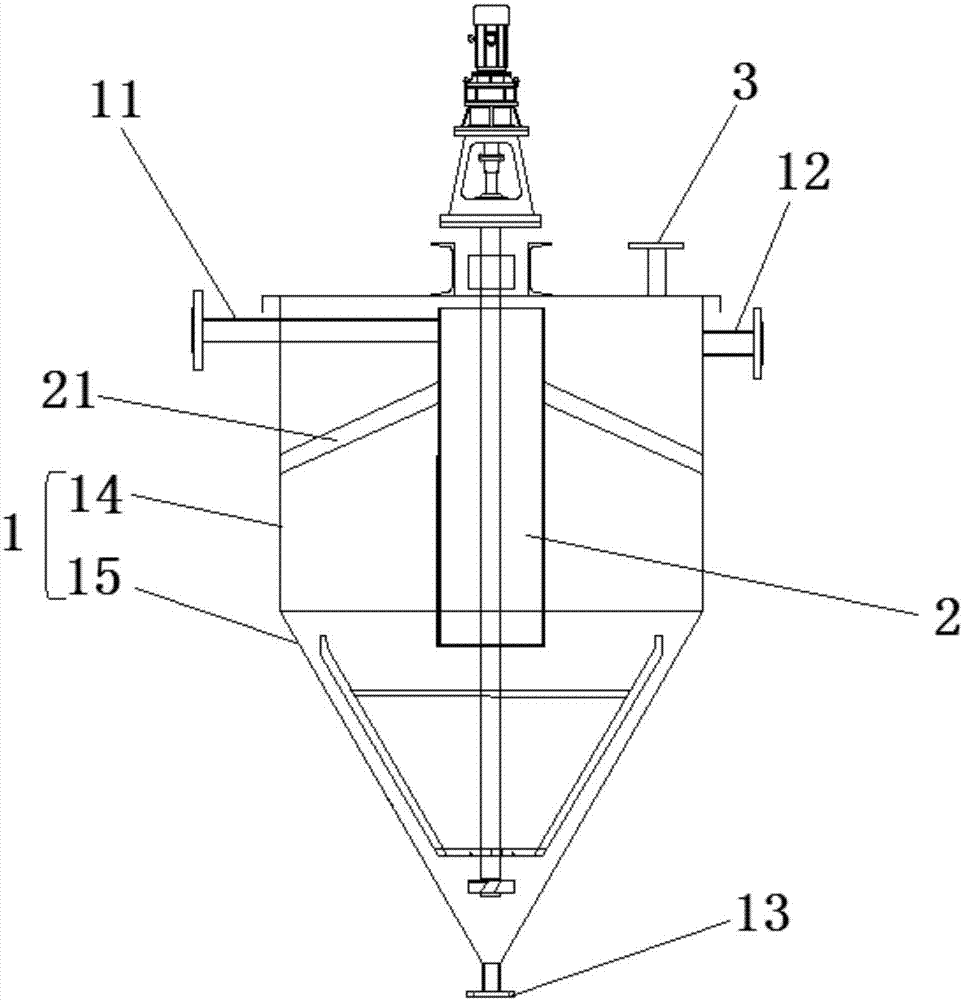
The evaporation kettle is an evaporation reaction vessel.It has an interlayer.the raw steam is passed into the outer layer of the kettle to heat internal material, and the secondary steam evaporated is cooled by the condenser. When the high brine is supersaturated, the Crystal slurry concentrate will be discharged from the lower end.The function of the evaporation kettle is to vaporize the distilled liquid, provide the necessary amount of ascending vapor in the column. The types of evaporation kettle are jacketed type, coiled type and tubular type.

There are two methods for crystallization of high brine: one is thermal crystallization, which is suitable for substances whose temperature has little effect on solubility. The “sunshine dry salt” in coastal areas is the method used. The other one is cooling the hot saturated solution method. This method is applicable to substances with increased temperature and increased solubility.

There are two methods for crystallization of high brine: one is thermal crystallization, which is suitable for substances whose temperature has little effect on solubility. The “sunshine dry salt” in coastal areas is the method used. The other one is cooling the hot saturated solution method. This method is applicable to substances with increased temperature and increased solubility.For example, in the salt lakes of the northern region, there is no crystal on the lake surface when the temperature is high in summer.When the temperature is lowered in winter, the mirabilite and other substances are separated from the salt lake.

It is a kind of driven fluid machinery, which relies on the mechanical energy input to increase the gas pressure and discharge the gas side by side. The increase of gas pressure is transformed by the velocity of the gas, that is, to make the inhaled air flow get a certain high speed, then slow it down, and let its momentum into the pressure of the gas rise, and then discharge.

The tube heat exchanger is one of the most widely used heat exchangers in chemical production and evaporation. It is mainly composed of casing, tube sheet, heat exchange tube, head, baffle plate etc. The required materials can be selected accordingly. The main pipes materials include: carbon steel, 304, 316L, 2205, TA2 and other materials.

The separator is a device for vapor-liquid separation of evaporated materials. Currently, the more commonly used equipments are: FC evaporator separator, OSLO evaporator separator, and DTB evaporator separator.

A centrifuge separator is a machine that uses centrifugal force to separate liquid and solid particles or components of a mixture of liquid and liquid. Centrifuges are primarily used to separate solid particles from the liquid.